Navigating the Regulatory Landscape: FDA and FTC Rules for Patient Influencer Marketing
The advent of social media has given rise to a new type of advocacy in the healthcare industry: patient influencers. These individuals navigate their healthcare journeys, often dealing with chronic illnesses, and share their experiences with a broader audience. In doing so, they create a community and become trusted voices that can impact healthcare decisions of others.
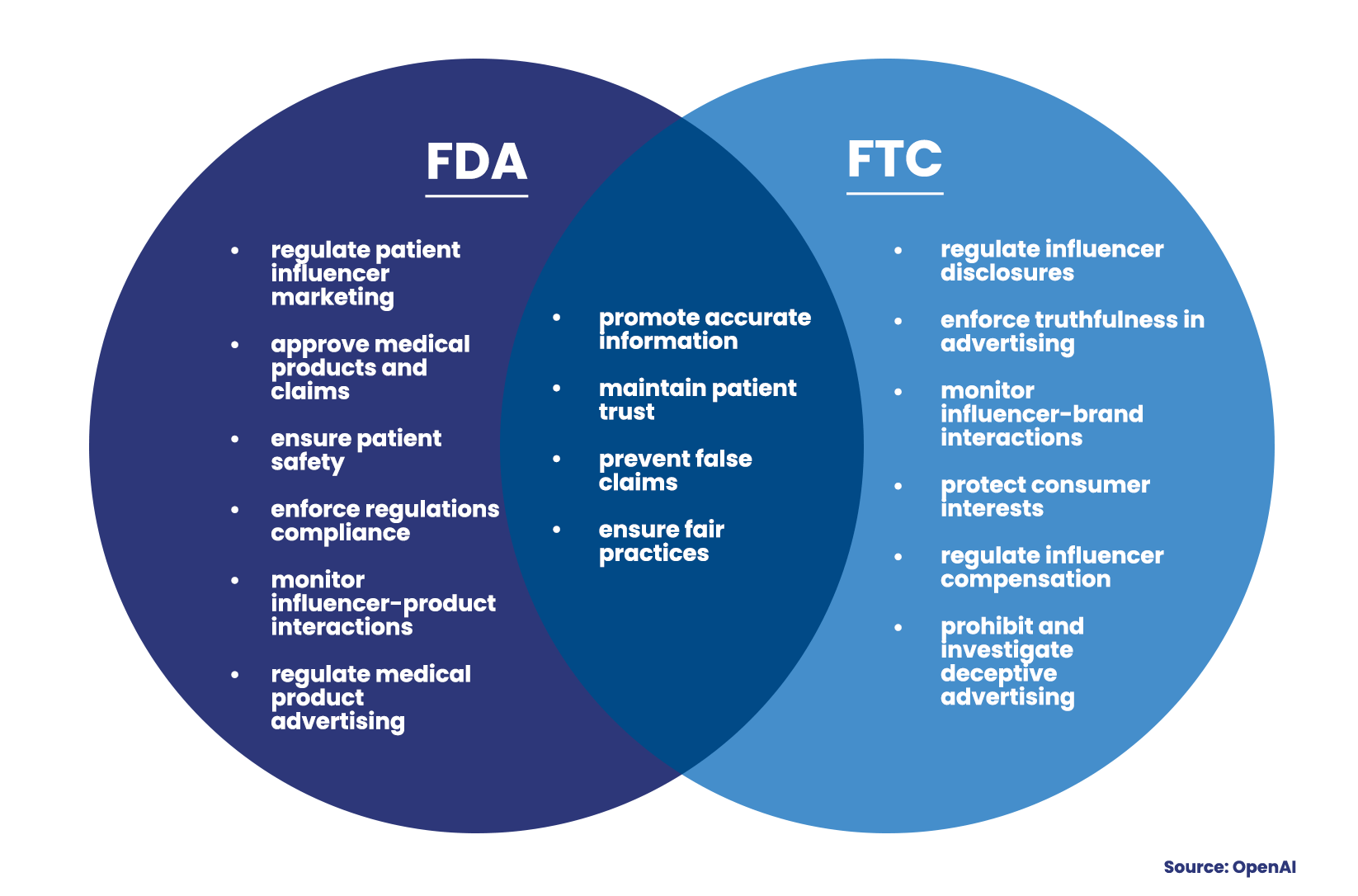
However, with this influence comes responsibility, particularly in the realm of regulatory compliance. Two key players oversee the rules and regulations for patient influencer marketing in the United States, namely the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC). The FDA’s primary concern is the accuracy and transparency of health-related information disseminated by influencers. It ensures that influencers fully disclose all associated information about the health products they promote, including potential risks and side effects.
Parallelly, the FTC manages the commercial side of patient influencer marketing. It mandates that influencers overtly disclose any compensation they receive for promoting health products. This level of transparency is typically achieved through the use of certain hashtags, such as #ad or #sponcon. The clear delineation of responsibilities between these two agencies ensures a comprehensive regulatory approach that values both patient safety and consumer protection.
Understanding the Importance of Compliance in Patient Influencer Marketing
Abiding by regulatory compliance is not just a legal necessity; it is a moral obligation that protects the safety and interests of patients. The significance of adhering to these guidelines was glaringly highlighted in the 2015 incident involving celebrity influencer Kim Kardashian. She endorsed a particular medication on her social media platform but failed to fully disclose the drug’s potential risks and side effects. This lack of transparency drew the attention of the FDA, resulting in a warning letter being sent to the drug company and the promotional post being promptly removed.
This incident served as a wake-up call for the industry, underscoring the importance of strict adherence to regulatory guidelines. Not only is compliance a legal requirement, but it also fosters trust between influencers and their followers, ensuring that patient safety is never compromised.
FTC’s Guidelines: A Comprehensive Resource for Social Media Influencers
Recognizing the significant role that social media influencers play in today’s digital landscape, the FTC published a document titled “Disclosures 101 for Social Media Influencers”. This comprehensive guide informs influencers about their legal obligations regarding disclosures and provides practical tips for making effective and transparent disclosures.
The guidelines are stringent, prohibiting influencers from discussing a product they haven’t personally used or making unfounded claims about a product. Additionally, influencers cannot fabricate claims about a product that would require scientific evidence. These guidelines serve to protect consumers, ensuring that they receive accurate and honest information from the influencers they trust.
Upholding Patient Safety with PhRMA’s Principles for Direct-to-Consumer Marketing
The pharmaceutical industry group, PhRMA, has also taken significant steps to ensure ethical marketing practices in healthcare. They have established a robust set of voluntary principles for direct-to-consumer marketing, which its members have agreed to uphold. These principles serve to reinforce the importance of patient safety and ethical conduct in marketing.
Additionally, both the FDA and FTC have announced their plans to carry out regular studies and evaluations. This ongoing monitoring aims to ensure adherence to regulations and continued compliance across the industry. This commitment to upholding regulatory standards reinforces the value placed on protecting patient interests.
Empowering Marketing Campaigns through Patient Influencers
Patient influencers bring a unique and authentic voice to the table. By integrating them into marketing campaigns, healthcare companies can reach a specific, targeted audience. These influencers can connect with patients who have similar medical conditions or are considering certain treatments, providing them with valuable insights and relevant information.
The credibility and trust that patient influencers have established with their followers
can significantly increase engagement and receptivity towards your product or service. This unique advantage stems from a shared understanding of the patient journey, making their endorsements authentic and relatable.
Moreover, forming collaborations with patient influencers can pave the way for long-term partnerships. With continued promotion of a product or treatment, these relationships can grow, leading to sustained awareness and interest in your offerings. As a result, patient influencers can significantly enhance the impact of your marketing campaigns.
Leveraging Patient Influencers for Digital Health Solutions
The realm of digital health solutions is a significant area where patient influencers can bring immense value. The FDA ensures that any information shared by influencers about digital health products is truthful, accurate, and includes any necessary risk information. By leveraging patient influencers in this area, companies can connect with a targeted audience of patients who are considering a particular digital health solution.
Not only do these influencers bring authenticity to their endorsements, but they also foster a sense of trust among potential users. It’s crucial to maintain clear records of interactions with patient influencers to ensure compliance with FDA requirements. Such meticulous record-keeping helps demonstrate a commitment to transparency and regulatory compliance.
The Role of Patient Influencers in Promoting Over-the-Counter Products
Over-the-counter (OTC) products are another area where patient influencers can play a vital role. As the FDA regulates the labeling and advertising of OTC products, influencers must ensure their endorsements are truthful and contain necessary risk information.
Their personal experiences with OTC products can provide a relatable narrative that resonates with other patients. As with digital health products, it’s essential to maintain clear records of interactions with influencers to ensure compliance with FDA regulations. This method of promotion not only helps reach your target audience effectively but also upholds regulatory standards, reinforcing the credibility of your products.
Patient Influencers: A Powerful Tool for Prescription Drug Promotion
Promotion of prescription drugs is another area where patient influencers can make a significant impact. The FDA closely regulates the promotion of prescription drugs, requiring truthful and comprehensive risk information in all endorsements.
Patient influencers can offer authentic and relatable narratives around their experiences with certain prescription drugs, making them a compelling resource to engage your target audience. The trust they’ve established with their followers enhances the effectiveness of their endorsements. As always, maintaining clear records of all interactions with influencers is paramount to ensure regulatory compliance.
Embracing the Potential of Patient Influencer Marketing
As we’ve seen, patient influencers can offer a myriad of benefits to healthcare marketing campaigns. They bring authenticity, relatability, and a powerful personal narrative that can resonate with your target audience. While navigating the regulatory landscape can be complex, adherence to FDA and FTC guidelines is paramount to ensure patient safety and trust.
By integrating patient influencers into your marketing strategy, you’re not just embracing a powerful promotional tool; you’re fostering a sense of community and trust among patients. You’re giving a voice to patient experiences, acknowledging their journeys, and valuing their insights.
So, consider harnessing the potential of patient influencer marketing. With careful compliance to regulatory standards, this approach can transform your marketing campaigns, creating a ripple effect of positive impacts in the healthcare landscape. After all, who better to endorse healthcare products and services than those who’ve experienced their benefits firsthand?
Embrace the potential. Empower the patients. Elevate your marketing campaigns. Let the power of patient influence guide your way to a more connected, trusting, and engaged audience.