Navigating Regulatory Compliance for Patient Influencer Marketing in Germany
In the ever-evolving healthcare marketing landscape, patient influencers are rapidly emerging as a potent force in Germany. With their unique ability to bridge the gap between health product companies and their target audiences, patient influencers are radically transforming the way health and wellness campaigns are conducted. This upsurge in popularity comes hand in hand with the critical need for a comprehensive understanding of the regulatory framework and strict adherence to compliance standards to ensure patient safety and the veracity of shared information.
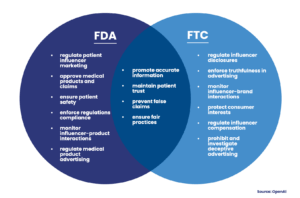
The Federal Institute for Drugs and Medical Devices (BfArM) and the Federal Cartel Office (FCO), the principal regulatory bodies in Germany, are charged with overseeing the rapidly growing field of patient influencer marketing. They ensure that all campaigns adhere to stringent guidelines that prioritize the accuracy of shared information and put patient safety at the forefront. This regulatory compliance framework is a vital component of Germany’s healthcare market, impacting several areas from telemedicine to digital therapeutics and remote patient monitoring.
Decoding the Regulatory Framework
Navigating the complex regulatory landscape for patient influencer marketing in Germany requires an emphasis on transparency and patient safety. The BfArM and the FCO mandate that the promotional nature of any content shared through patient influencers must be apparent to an average user. This regulation implies that any posts or videos carrying a promotional intent must prominently mention “Werbung” or “Anzeige” at the beginning and in their descriptions.
The reliance on hashtags such as “#sponsored” or “#ad” might not be sufficient to meet the necessary legal standards as defined by the courts. Therefore, employees in the pharmaceutical sector, biotech companies, medtech firms, healthtech organizations, and health insurance providers must be diligent in their compliance with these regulations to avoid potential legal issues. Moreover, organizations engaging in B2C marketing need to be mindful of these guidelines when planning their campaigns and forging agency partnerships. They must ensure that their marketing efforts align with the expectations of the regulatory bodies, contribute positively to the patient journey, and address any unmet needs in the market.
Patient Influencer Marketing in the Digital Health Arena
The digital health sector, a vibrant and rapidly growing segment of the healthcare industry, is increasingly leveraging patient influencer marketing to extend its reach and impact. The BfArM plays a crucial role in regulating this space, ensuring that the promotion of digital health solutions, including Digital Health Applications (DiGA), aligns with the regulatory guidelines. This means that any information shared by patient influencers about digital health solutions or DiGA must be truthful, accurate, and include any necessary risk information associated with the product.
Companies operating in the digital health space can rely on these regulations to ensure the veracity of the information shared by patient influencers. Furthermore, these companies are required to maintain detailed records of their interactions with patient influencers and must be prepared to provide this information to the BfArM upon request. This requirement helps ensure compliance with regulations and fosters a more transparent and trustworthy digital health ecosystem. It also underlines the importance of sentiment analysis, focus group studies, and patient surveys in gauging the effectiveness of patient influencer marketing campaigns and understanding the evolving market trends in the digital health sector.
Navigating Compliance in the Promotion of Over-the-Counter (OTC) Products
Navigating the regulatory landscape of promoting over-the-counter (OTC) products in Germany presents its unique set of challenges and opportunities. The Federal Cartel Office (FCO) regulates this domain, and adherence to its regulations is mandatory. The promotion of OTC products is allowed, providing it respects the stipulations set forth by the FCO. These stipulations include ensuring that all information shared about the product is factual and accurate, and includes necessary risk information.
The FCO further requires that any promotions for OTC products must unmistakably identify themselves as such. Consequently, this means that the relationship between the patient influencer and the brand/vendor must be disclosed transparently, and any compensation or agreements between them must be clearly mentioned. This stipulation underscores the importance of maintaining a clear and honest dialogue with patient influencers, prioritizing transparency, and fostering an environment of trust.
Pharmaceutical employees engaged in marketing campaigns involving patient influencers must maintain meticulous records of their interactions with them. The FCO may request these records at any time, and being prepared to provide this information is a critical component of regulatory compliance. This requirement also stresses the importance of staying abreast of the specific guidelines set by the FCO, and ensuring that all advertisements or promotions for OTC products follow the regulations set by the FCO.
Regulatory Compliance in Prescription Drugs Promotion
The promotion of prescription drugs in Germany is subject to strict regulations, primarily overseen by the BfArM. The BfArM’s role in this context is to ensure that accurate and truthful information about prescription drugs is disseminated to patients. Companies operating in the pharmaceutical space can trust that the information shared by patient influencers complies with these regulations and includes any necessary risk information associated with the product.
Pharmaceutical companies must also maintain thorough records of their interactions with patient influencers. These records could be requested by the BfArM at any time to ensure regulatory compliance. This process underscores the importance of building trust and maintaining transparency in the pharmaceutical industry. In addition, it shows the significance of market research, healthcare market reports, and pharma market insights in informing companies’ strategic decisions and ensuring they stay ahead of the curve in the evolving healthcare landscape.
The Role of the German High Court in Influencer Marketing
In addition to the regulations set by BfArM and the FCO, the German High Court (BGH) has made some crucial rulings that impact influencer marketing. Notably, it ruled that influencers can refer to companies on the internet using features built into social networks, such as “tags” in photos on Instagram, which redirect users to manufacturers or brands profiles, without this being considered as advertising.
This ruling has significant implications for companies and influencers alike, as it allows them to connect with their audience in a more natural and authentic way. However, it’s crucial to note that if the influencer directly links to the brand’s website or Instagram account, this would be considered advertising. This distinction underscores the importance of understanding the fine line between brand advocacy and advertising, and the necessity for companies and influencers to navigate this space responsibly and ethically.
In conclusion, understanding and adhering to the regulatory compliance framework for patient influencer marketing in Germany is critical. Companies must ensure they navigate this landscape effectively, keeping abreast of the latest rulings and guidelines, and maintaining an open and transparent relationship with their influencers. By doing so, they can leverage the power of patient influencers in a responsible and effective manner, driving brand affinity, enhancing patient experience, and contributing to the growth and development of the healthcare industry.